Mosunetuzumab▽ Plus Polatuzumab Vedotin has Promising Efficacy and a Favorable Safety Profile in Patients with Relapsed/Refrac

Raul Cordoba, MD, PhD on Twitter: "#ASH19 Mosunetuzumab induces CR in poor prognosis NHL patients including those who are resistant to or relapsing after #CART therapies #lymsm. Data coming from the first
Mosunetuzumab in Combination with Lenalidomide Has a Manageable Safety Profile and Encouraging Activity in Patients with Relapse

EU approves Roche's Lunsumio to treat relapsed or refractory follicular lymphoma | Companies | POST Online Media
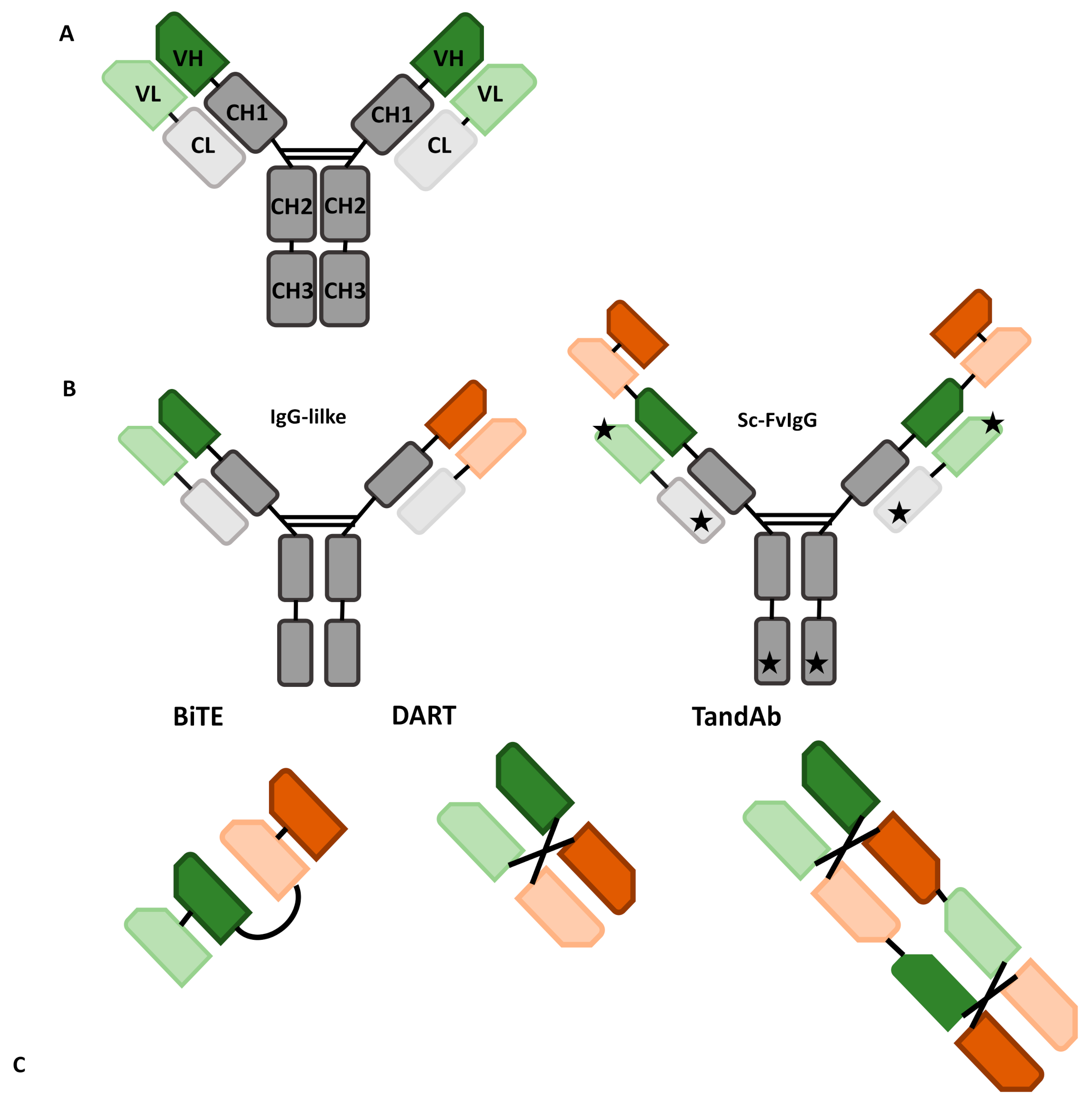
Antibodies | Free Full-Text | The Role of Bispecific Antibodies in Non-Hodgkin’s Lymphoma: From Structure to Prospective Clinical Use

ASH 2022: Pharmacodynamic Biomarkers of Mosunetuzumab Efficacy and Safety in Patients with Relapsed/Refractory Non-Hodgkin Lymphoma: Results from a Phase I/II Study

Mosunetuzumab Monotherapy Is an Effective and Well-Tolerated Treatment Option for Patients with Relapsed/Refractory (R/R) Follicular Lymphoma (FL) Who Have Received ≥2 Prior Lines of Therapy: Pivotal Results from a Phase I/II Study -

Safety and efficacy of mosunetuzumab, a bispecific antibody, in patients with relapsed or refractory follicular lymphoma: a single-arm, multicentre, phase 2 study - The Lancet Oncology

A Safety, Efficacy and Pharmacokinetic Study of BTCT4465A (Mosunetuzumab) as a Single Agent and Combined With

SOHO 2022: Mosunetuzumab Monotherapy Is an Effective and Well-Tolerated Treatment Option for Patients With Relapsed/Refractory (R/R) Follicular Lymphoma (FL) Who Have Received ≥2 Prior Lines of Therapy: Pivotal Results From a Phase

FDA Approves Lunsumio (mosunetuzumab-axgb) for the Treatment of Relapsed or Refractory Follicular Lymphoma








