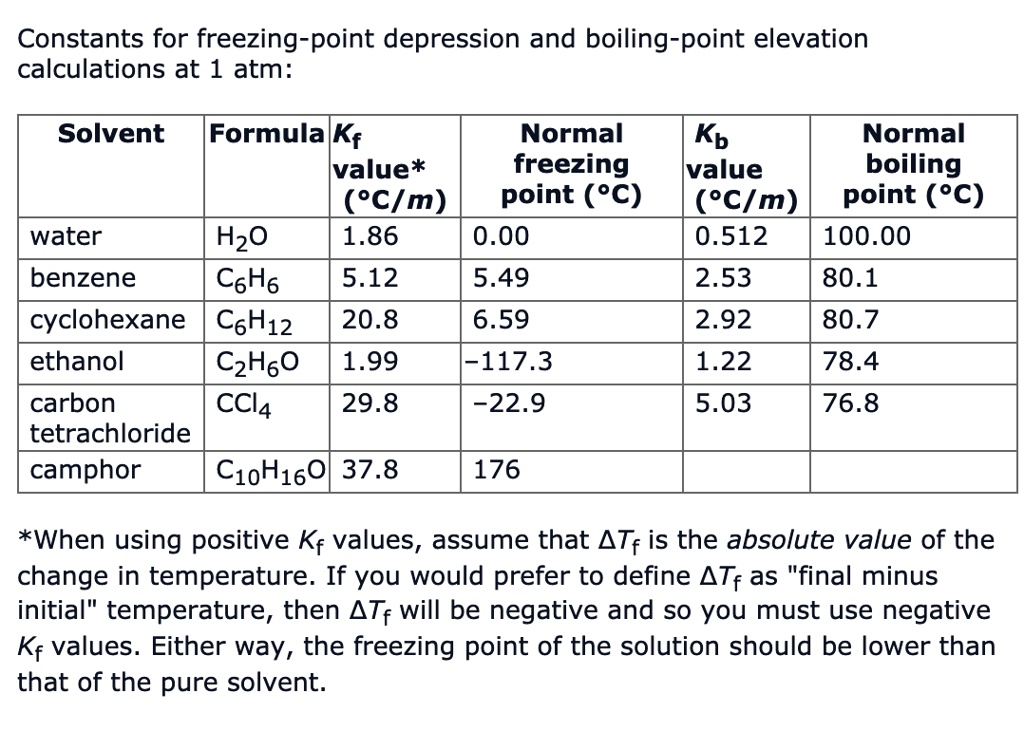
Calculate the lattice energy of potassium fluoride, KF, using the Born-Haber cycle. Use thermodynamic data to obtain the enthalpy changes for each step. | Homework.Study.com

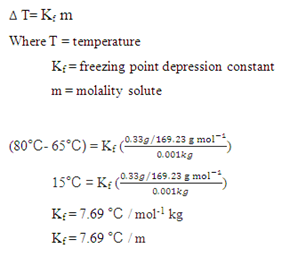
Calculate the mass of ascorbic acid to be dissolved in 75 g acetic acid to lower its melting point by 1.5 ̊C , Kf = 3.9 K kg / mol – The Unconditional Guru

what mass of NaCl must be dissolved in 65 g of water to lower the frezing point by 7 50 - Chemistry - Solutions - 13869393 | Meritnation.com
Q6 Calculate the freezing point of an aqueous solution containing 10.50 g of MaBrz in 200 gof water (molar mass of MgBr2 184 g mol 1 ). Given Kf for water = 1.86 KKg mor

After you preform your experiment, you determine that the Kf value for naphthalene is 6.9 . You are using 10g of naphthalene and added 1.0 g of your unknown. The the freezing

After you preform your experiment, you determine that the Kf value for naphthalene is 6.9 . You are using 10g of naphthalene and added 1.0 g of your unknown. The the freezing
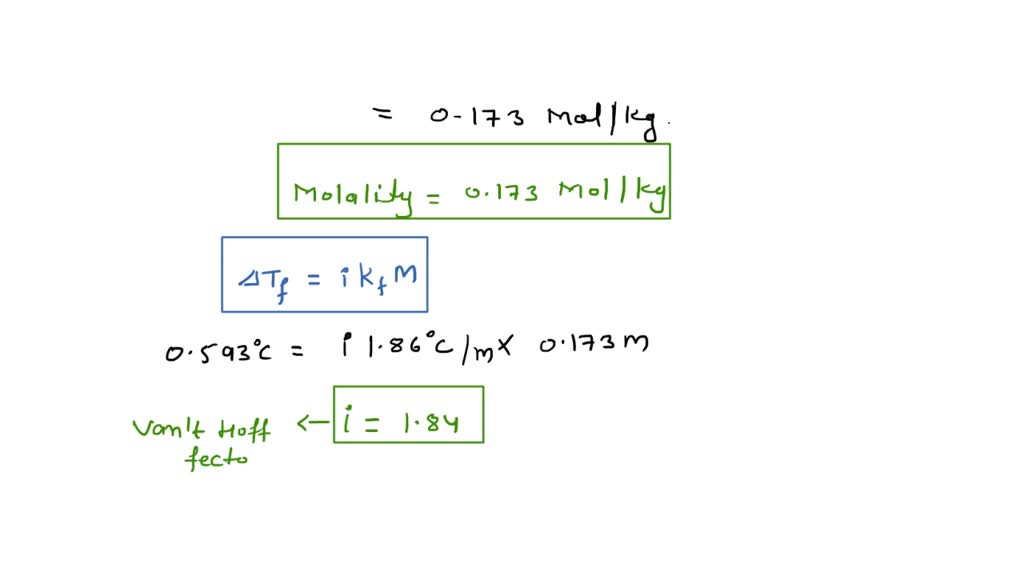
SOLVED: 1) Determine the freezing point for a solution with a concentration of 0.100 m K2SO4 in acetic acid (Tf = 16.6 oC, Kf = -3.90 oC/m). a.) Calculate the freezing point

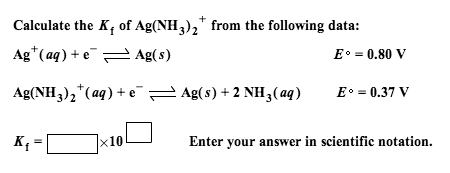
Calculate Complex Ion Equilibria Using the Small x Approximation for Large Kf | Chemistry | Study.com
![Calculate the depression in the freezing point of water when 10 g of CH3CH2CHClCOOH is added to 250 g of water. [ Ka = 1.4 × 10^-3, Kf = 1.86 K kg mol^-1 ] Calculate the depression in the freezing point of water when 10 g of CH3CH2CHClCOOH is added to 250 g of water. [ Ka = 1.4 × 10^-3, Kf = 1.86 K kg mol^-1 ]](https://i.ytimg.com/vi/zGfIbhioFZ0/maxresdefault.jpg)
Calculate the depression in the freezing point of water when 10 g of CH3CH2CHClCOOH is added to 250 g of water. [ Ka = 1.4 × 10^-3, Kf = 1.86 K kg mol^-1 ]

Colligative Properties - Boiling Point Elevation, Freezing Point Depression & Osmotic Pressure - YouTube

Calculate the freezing point of a solution when 3 g of CaCl2(M = 111 g mol^-1) was dissolved in 100 g of water.assuming CaCl2 Undergoes complete ionisation (Kf for water = 1.86 K kg mol^-1)