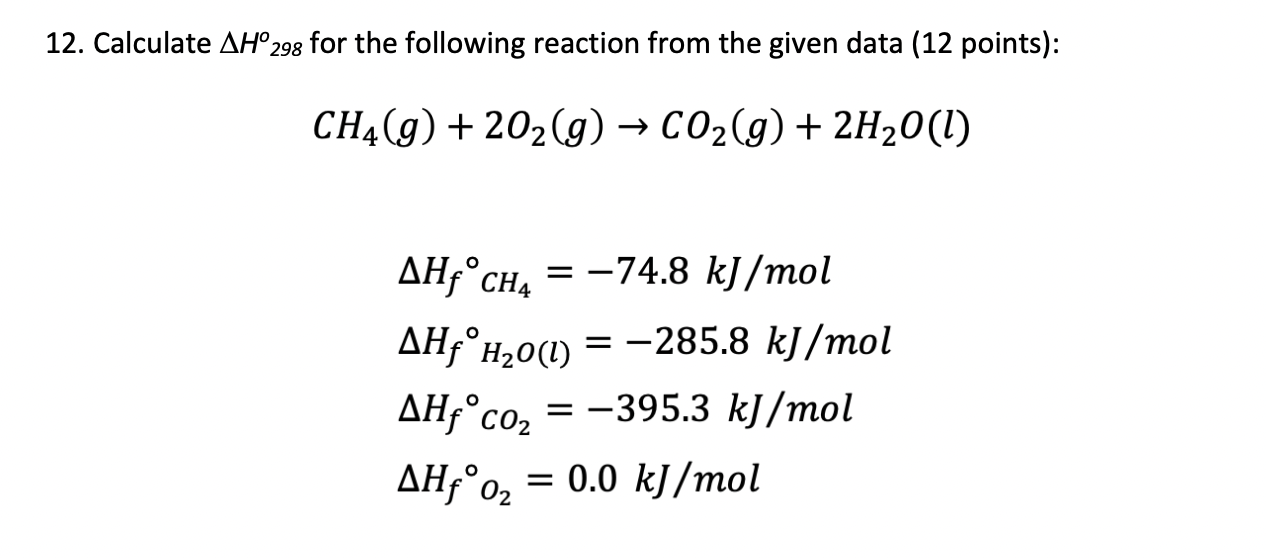
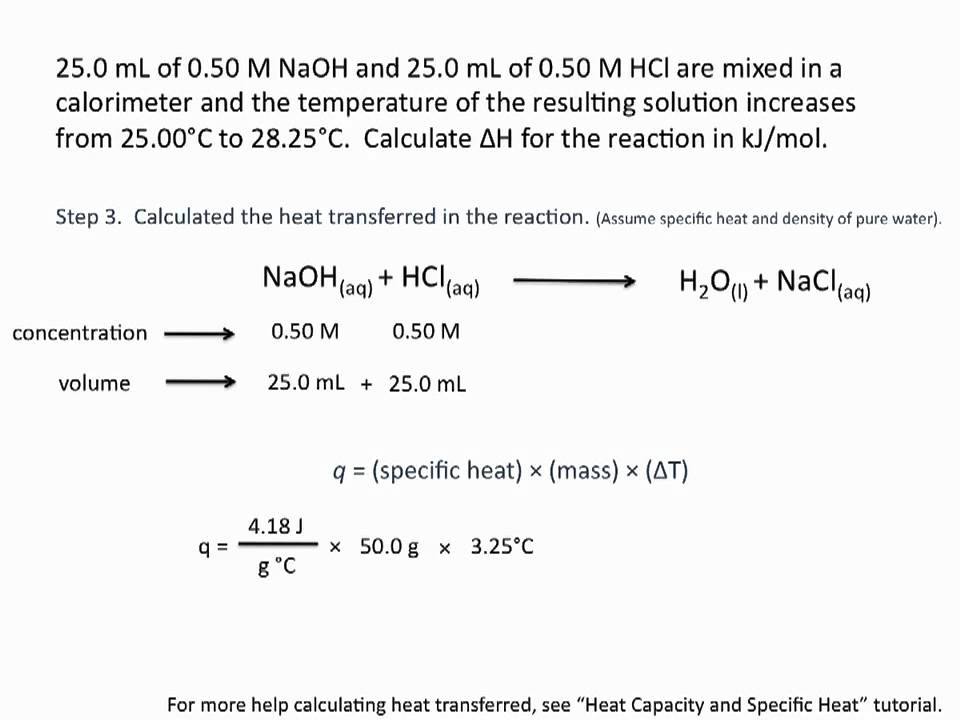
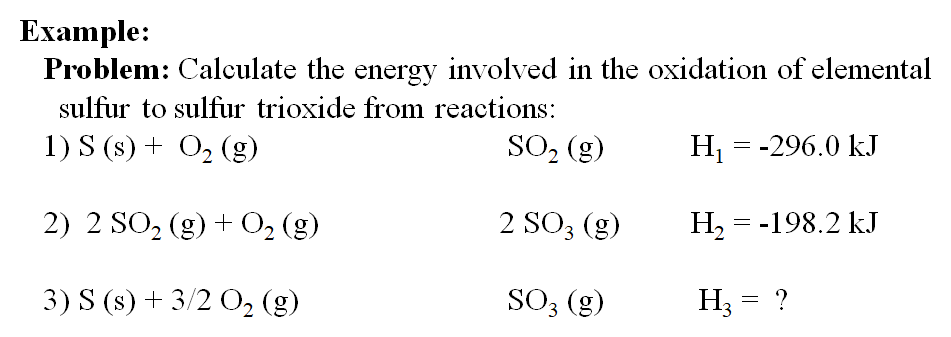Calculate enthalpy for formation of ethylene from the following data:(I) C(graphite) + O2 (g) → CO2 (g); Δ H = - 393.5 kJ (II) H2(g) + 12 O2 (g) → H2O(l); Δ

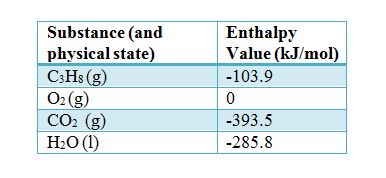
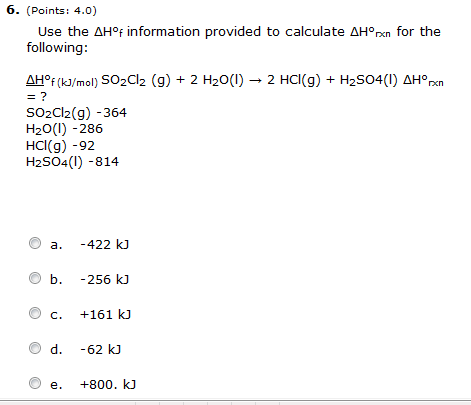
OneClass: Calculate the standard enthalpy change, Delta H degree rxn, in kJ for the following chemica...
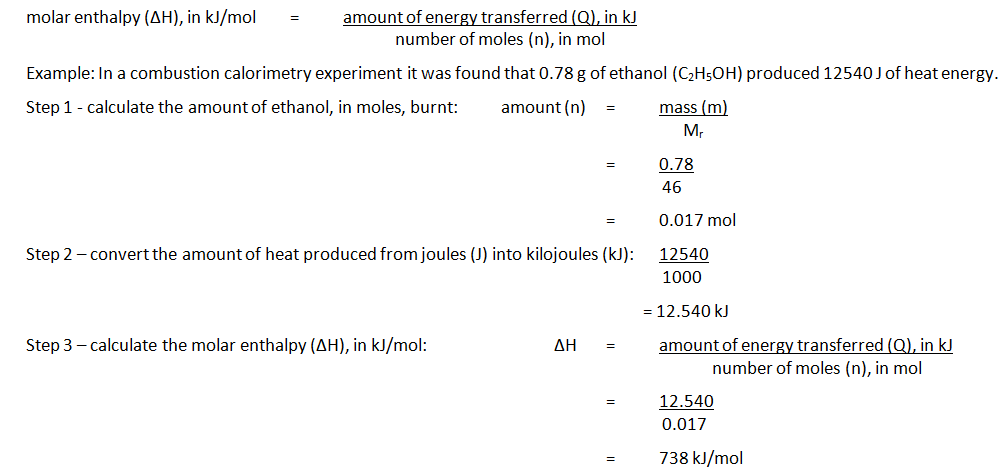
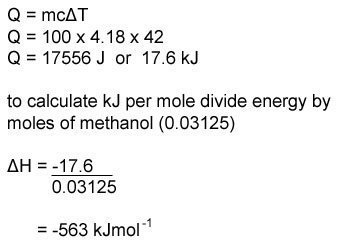
3:04 calculate the molar enthalpy change (ΔH) from the heat energy change, Q - TutorMyself Chemistry
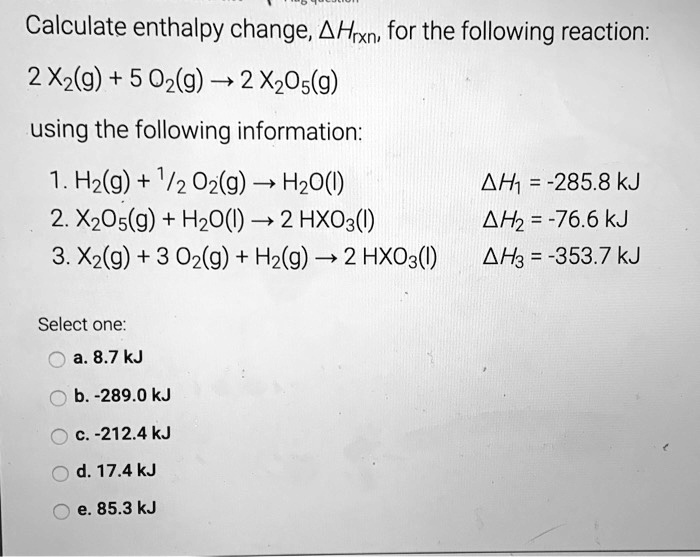
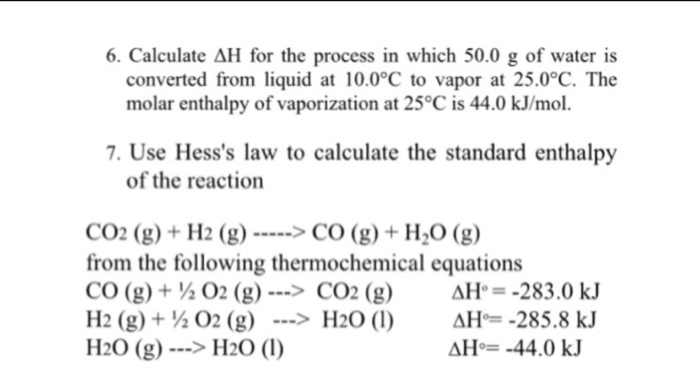
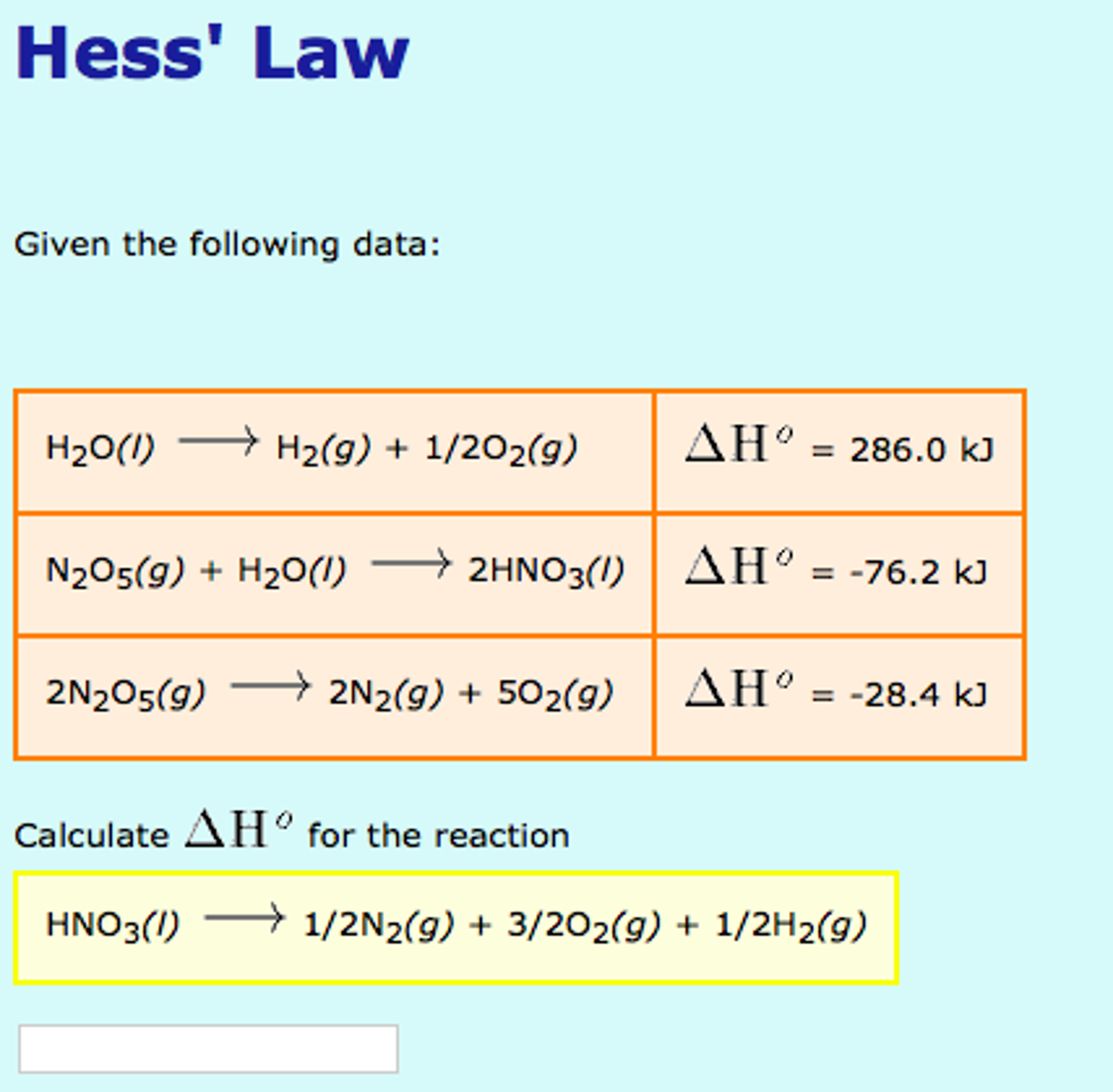
SOLVED: Calculate enthalpy change, AHrxn for the following reaction: 2 X2(g) + 5 02(g) 2 XzO5(g) using the following information: Hz(g) + 1/2 Oz(g) HzO() AH1 =-285.8kJ 2. XzOs(g) + HzO() 2